Excerpted from:
GE Power & Water
Water & Process Technologies
Handbook of Industrial Water Treatment
(https://www.gewater.com/handbook/index.jsp)
pH Control
Download a PDF version of this excerpt by clicking here
Maintenance of proper pH throughout the boiler feedwater, boiler, and condensate systems is essential for corrosion control. Most low-pressure boiler system operators monitor boiler water alkalinity because it correlates very closely with pH, while most feedwater, condensate, and high-pressure boiler water requires direct monitoring of pH.
Control of pH is important for the following reasons:
- Corrosion rates of metals used in boiler systems are sensitive to variations in pH
- Low pH or insufficient alkalinity can result in corrosive acidic attack
- High pH or excess alkalinity can result in caustic gouging/cracking and foaming, with resultant carryover
Speed of oxygen scavenging reactions is highly dependent on pH levels
The pH or alkalinity level maintained in a boiler system depends on many factors, such as system pressure, system metals, feedwater quality, and type of chemical treatment applied.
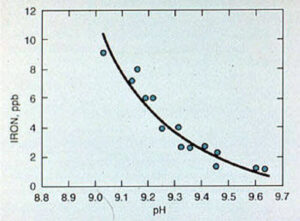
The corrosion rate of carbon steel at feedwater temperatures approaches a minimum value in the pH range of 9.2-9.6 (see Figure 11-9). It is important to monitor the feedwater system for corrosion by means of iron and copper testing. For systems with sodium zeolite or hot lime softened makeup, pH adjustment may not be necessary. In systems that use deionized water makeup, small amounts of caustic soda or neutralizing amines, such as morpholine and cyclohexylamine, can be used.
Figure 11-9. Iron corrosion product release from carbon steel in boiler feedwater.
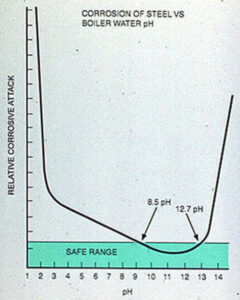
In the boiler, either high or low pH increases the corrosion rates of mild steel(see Figure 11-10). The pH or alkalinity that is maintained depends on the pressure, makeup water characteristics, chemical treatment, and other factors specific to the system.
Figure 11-10. High or low boiler water pH corrodes boiler steel.
The best pH for protection of copper alloys is somewhat lower than the optimum level for carbon steel. For systems that contain both metals, the condensate and feedwater pH is often maintained between 8.8 and 9.2 for corrosion protection of both metals. The optimum pH varies from system to system and depends on many factors, including the alloy used (see Figure 11-11).
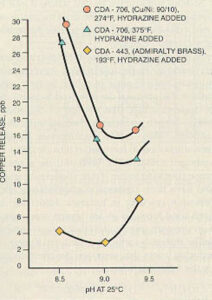
Figure 11-11. Average copper release as a function of pH shows optimum pH in range of 8.8 to 9.2 for different copper-based alloys. (Courtesy of the Electric Power Research Institute.)
To elevate pH, neutralizing amines should be used instead of ammonia, which (especially in the presence of oxygen) accelerates copper alloy corrosion rates. Also, amines form protective films on copper oxide surfaces that inhibit corrosion.